The MedTech industry is governed by strict regulations that require full traceability.
Yet most early-stage MedTech companies still work with a tool zoo. Their records are scattered across several systems to manage quality, supply chain, compliance, and core business processes.
As a result, information isn’t tracked properly, and companies risk both legal nonconformity and fragmented operations.
What’s needed is a system that can connect regulated processes like quality management and compliance with everyday operations, such as supply chain and sales.
Odoo can do both. See how MedTech companies can cover all their key operation processes with Odoo, while also adhering to MDR, FDA, and ISO demands.
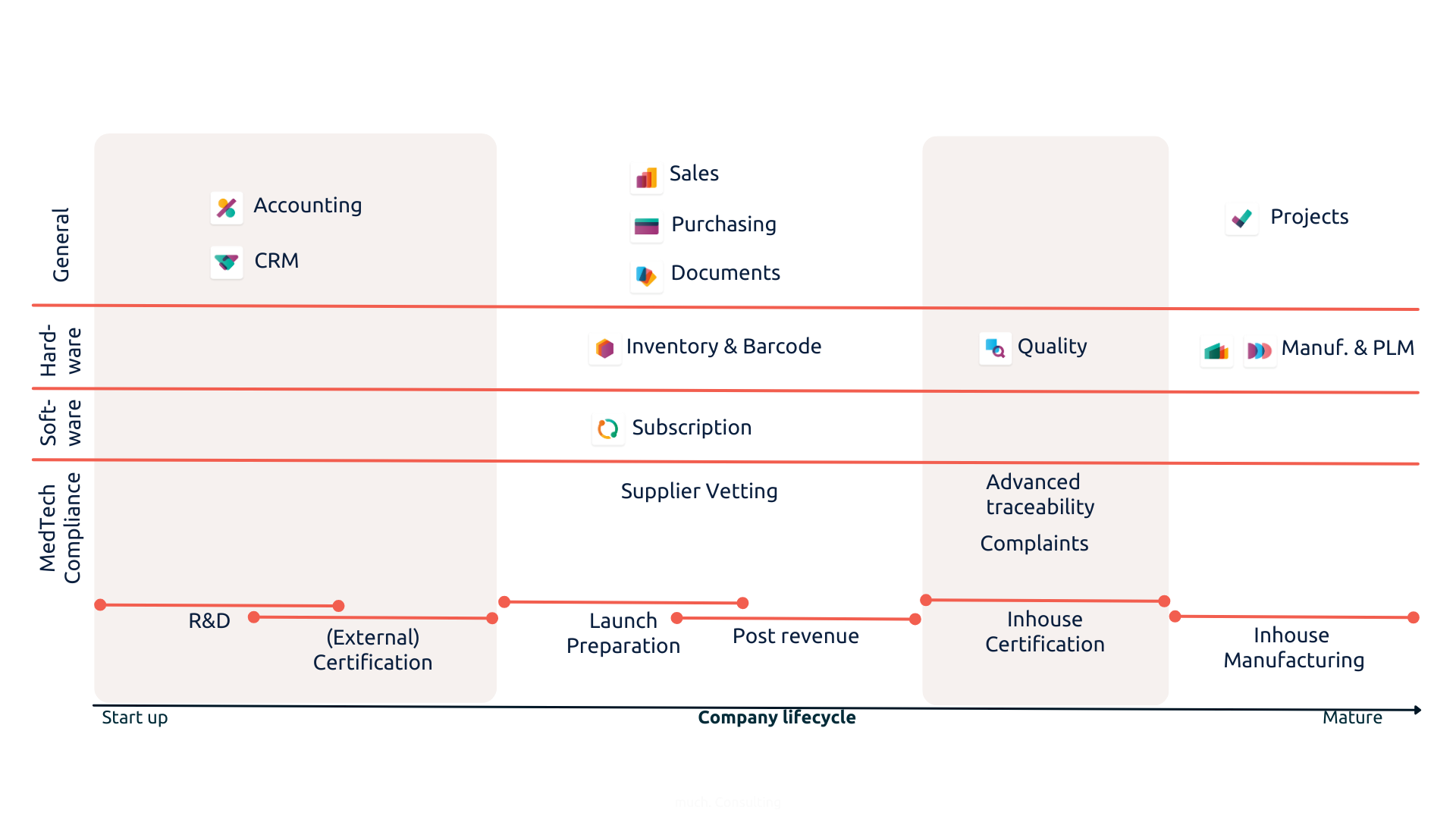
The entire lifecycle of a MedTech company can be supported by Odoo
Table of contents
Strict regulations make it difficult to run MedTech operations
MedTech struggles to find an ERP that fits all its needs
Odoo covers 30+ key processes of MedTech out of the box
Odoo can be extended to meet traceability needs & regulations
Strict regulations make it difficult to run MedTech operations
Any MedTech company that wants to scale is bound by two major legal frameworks: MDR in Europe and FDA clearance in the United States.
MDR (Medical Device Regulation) governs all devices sold in the EU. It requires continuous traceability, rigorous documentation, and ongoing post-market surveillance. Under MDR, companies must maintain compliance throughout the device’s lifecycle, with audits verifying this on a regular basis.
FDA approval governs all devices sold in the US. While equally strict, the focus is different: It puts stronger emphasis on pre-market approval, extensive safety testing before launch, and clear evidence of efficacy.
The difference between the two is mainly in timing and focus: Europe insists on continuous oversight throughout a device’s life. The U.S. requires rigorous validation before the products are allowed on the market.
Alongside these legal requirements, regulators on both sides of the Atlantic also rely on ISO standards, the international benchmark for quality management.
ISO 9001 defines general rules for process control and oversight.
ISO 13485 builds on this and adapts it for medical devices, requiring documented procedures, risk management, and lifecycle records.
MedTech struggles to find an ERP that fits all its needs
The main challenge for early-stage MedTech companies is managing two worlds at once.
On one side are the strict requirements of regulators: every part tracked, every update validated, every supplier certified.
On the other side are the needs of a growing business: sales, inventory, customer management, and operations that need to move fast.
Most ERPs lean one way or the other.
General systems may handle day-to-day business but lack the traceability required for audits.
Designated quality management systems may cover compliance but offer little support for commercial operations.
As a result, companies often end up juggling multiple tools, which only increases complexity and makes it more difficult to keep track of audits for mandatory compliance certifications.
Finding an ERP that meets all the needs of a MedTech company is difficult because of challenges like:
- Strict validation requirements: Software must be tested and documented in extreme detail to prove safety. Each re-validation slows down releases and discourages improvements
- Rigid certification processes: Systems must remain compliant as they evolve, but most can’t handle updates without triggering new audits. As a result, companies often leave legacy systems in place long after they should have been replaced
- Complex integrations: Software must connect to hospital IT, devices, and standards like HL7, FHIR, and DICOM. Each integration requires compliance checks, delaying rollout and scaling.
Odoo covers 30+ key processes of MedTech out of the box
To cover all their specific processes and tight industry regulations, MedTech companies, on average, work with more than 20 different tools:
- CAD software for design
- Clinical study tools for trials
- Logistics systems to manage production
- Office software to keep track of documentation
- Specialised compliance tools to stay within legal requirements
Each process is in a different place, making audits slow and traceability incomplete.
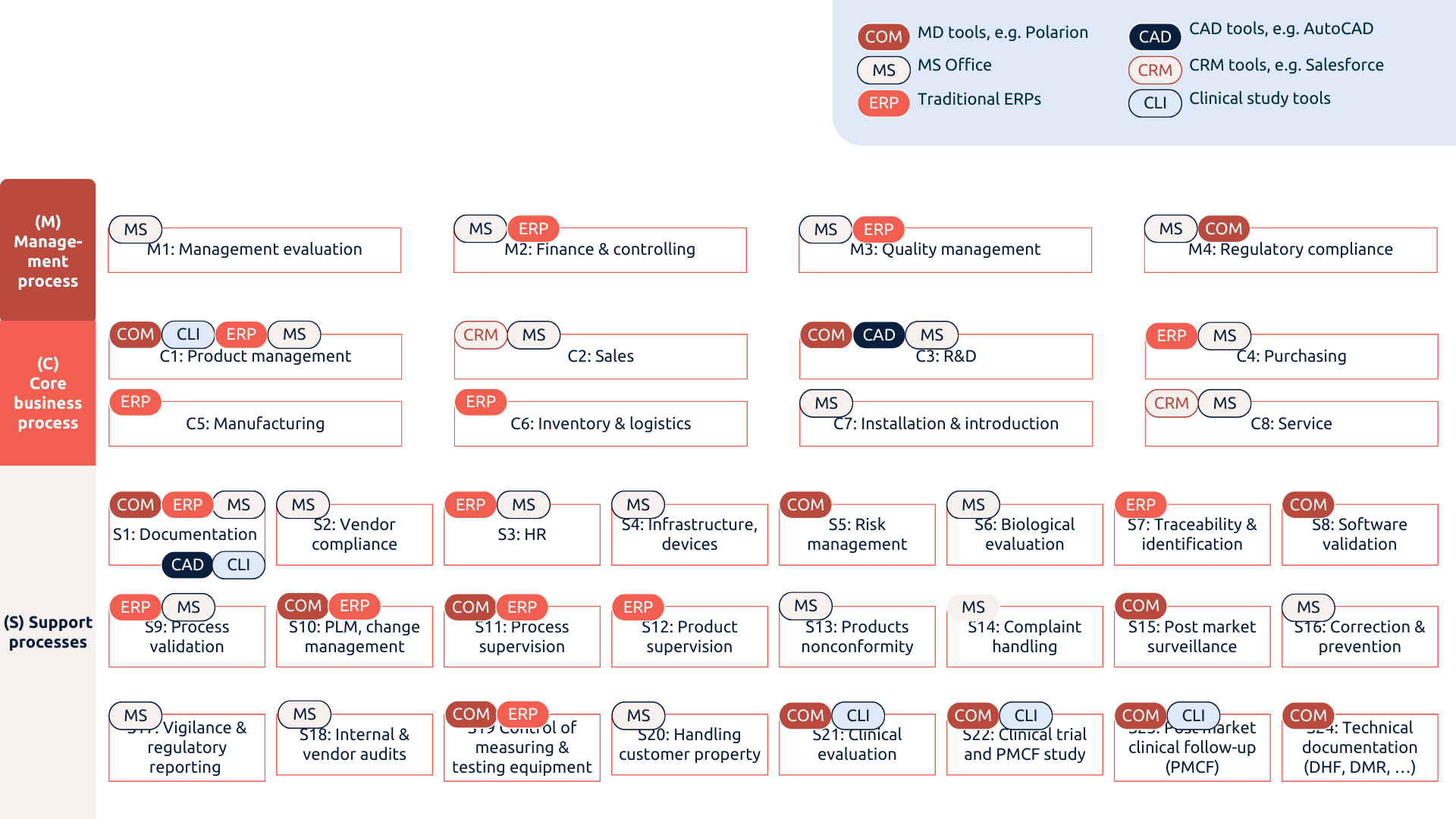
MedTech companies have their processes scattered over dozens of systems.
Odoo can replace most of these systems in one environment.
Out of the 36 key processes identified in MedTech, Odoo covers more than 30 out of the box, including:
- Management processes such as finance, quality, and regulatory compliance
- Operational processes such as product management, sales, inventory, purchasing, manufacturing, and service
- Support processes such as documentation, vendor compliance, HR, infrastructure, process validation, complaint handling, and audits.
Some MedTech processes still require external systems: for example, CAD design, clinical study tools, and specialist risk evaluation.
Still, thanks to its open-source nature, Odoo can easily integrate via API with these systems, to keep all operational and compliance data in one place.
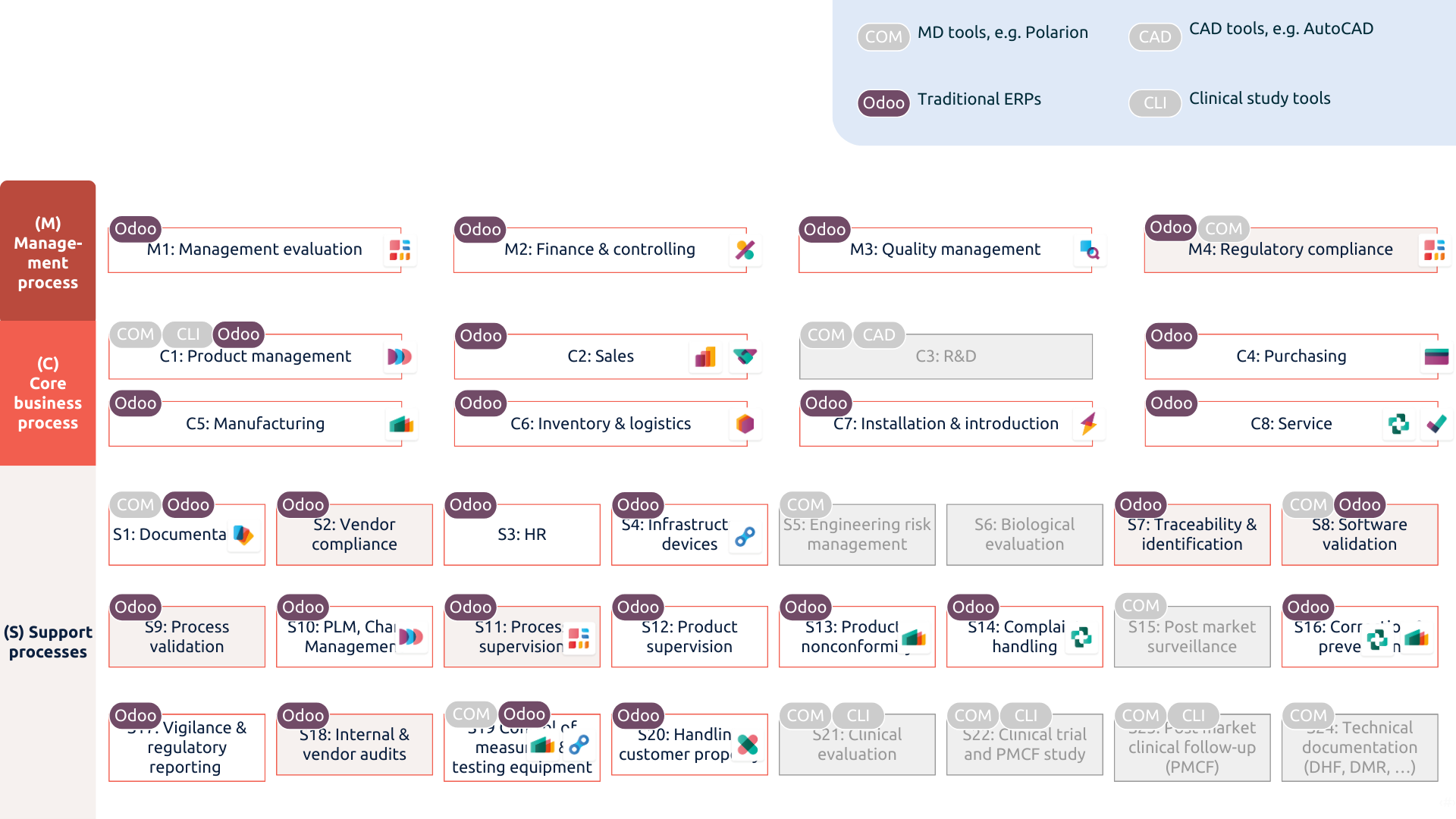
Odoo tackles most of the challenges faced by MedTech companies and integrates with specific software to keep compliance and operational requirements running smoothly.
Odoo can be extended to meet traceability needs & regulations
Odoo provides a solid foundation for a MedTech system, but there are still core requirements that are not covered by its Standard version.
That’s why we developed a vertical solution for MedTech that builds on top of Odoo ERP and extends the system with all the needed features:
Enhanced traceability
Our solution builds on standard tracking with full lifecycle transparency for every product, part, and update. We connect repairs, updates, and service records directly to serial numbers, ensuring no detail is missed.
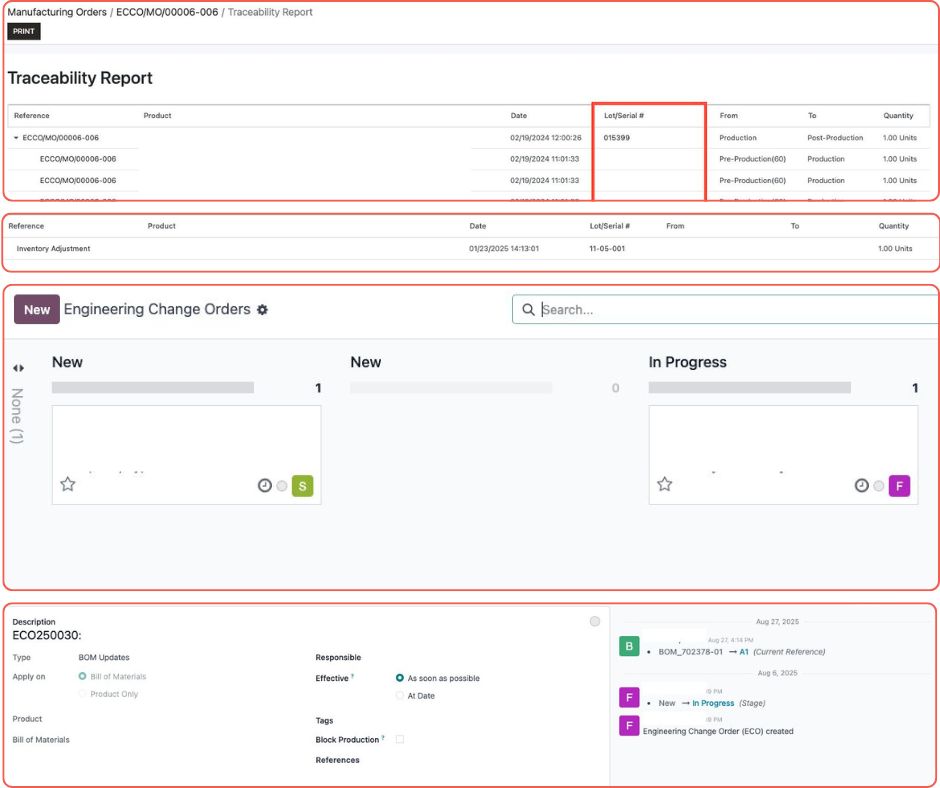
Vendor compliance management
We help you automate supplier certification tracking, manage risk groups, and prevent non-compliant purchases to simplify procurement and ensure adherence to regulations.
Custom features for MedTech
We can fill the gaps in Odoo standard with tools designed for risk management, batch recalls, and compliance reporting tailored to the medical device industry
Regulatory dashboards
You can monitor compliance in real-time with tailored dashboards that map specific regulatory requirements (MDR, FDA) to your processes.
Automated testing & validation
Our solution optimises process validation with automated test cases, ensuring regulatory compliance for every software version update.
Examples: how Odoo works for real MedTech use cases
MedTech companies face very different challenges depending on their product type and risk class.
See how real MedTech projects have applied Odoo for their specific use cases:
Traceability for X-ray device manufacturing
The problem
A manufacturer of X-ray machines needed full traceability of parts and suppliers to meet MDR audit standards.
The Odoo solution
Product lifecycle management and inventory tracking were implemented in Odoo, with serial numbers linked directly to supplier certifications.
The benefits
Instead of spending weeks compiling records for audits, the company now generates complete traceability reports directly from the system in hours.
End-to-end compliance for hearing implants
The problem
The development of hearing implants combined hardware and embedded software, making compliance complex across multiple components.
The Odoo solution
Odoo provided version control for both hardware and software, integrated with supplier management and production processes.
The benefits
Every release can be traced from design through manufacturing and testing, satisfying both MDR and FDA requirements in a single system.
Documentation control for pacemakers
The problem
A pacemaker manufacturer faced strict documentation requirements as a class III device producer. Every step, approval, and change had to be recorded and signed off.
The Odoo solution
Quality management workflows were set up in Odoo with electronic approvals embedded in daily operations.
The benefits
The company now maintains a complete device history without relying on external spreadsheets, simplifying audits and inspections.
Unified processes for vision-controlled wheelchairs
The problem
A company producing vision-controlled wheelchairs struggled with disconnected R&D, procurement, and production data.
The Odoo solution
Odoo consolidated BOM management, supplier compliance, and manufacturing orders into one system.
The benefits
Information across departments is now aligned, reducing manual consolidation and ensuring a clear audit trail for regulators.
Software validation for ICU monitoring systems
The problem
A MedTech firm building ICU monitoring systems with real-time data and alarms needed to validate software at every release.
The Odoo solution
Automated test protocols were linked to development, release, and quality modules in Odoo.
The benefits
Re-validation is now optimised with every software update, reducing delays and ensuring compliance in a critical care environment.
Our Managing Martner, Simon Stappen, spoke about how to address the most common MedTech challenges at Odoo Experience 2024.
Check out his breakdown of how MDR, FDA, and ISO 13485 requirements can be addressed in Odoo standard with our custom solutions.
Want to implement Odoo for your MedTech company?
Talk to our Odoo consultants with expertise in MedTech to connect compliance, traceability, and operations in one system.